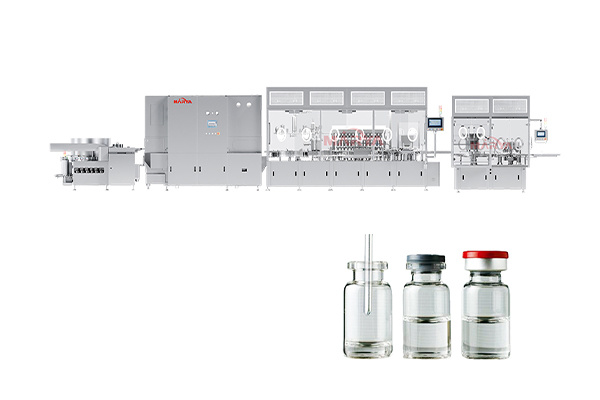
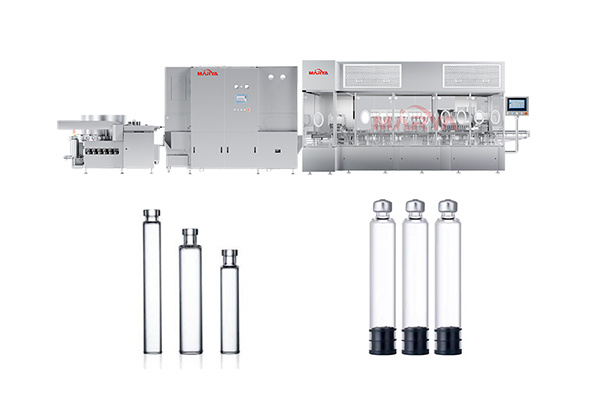
Products




Pharmaceutical Multi-effect Distilled Water Machine
- Working Principle
- Core Features
- Technical Parameters
- Application Industry
-
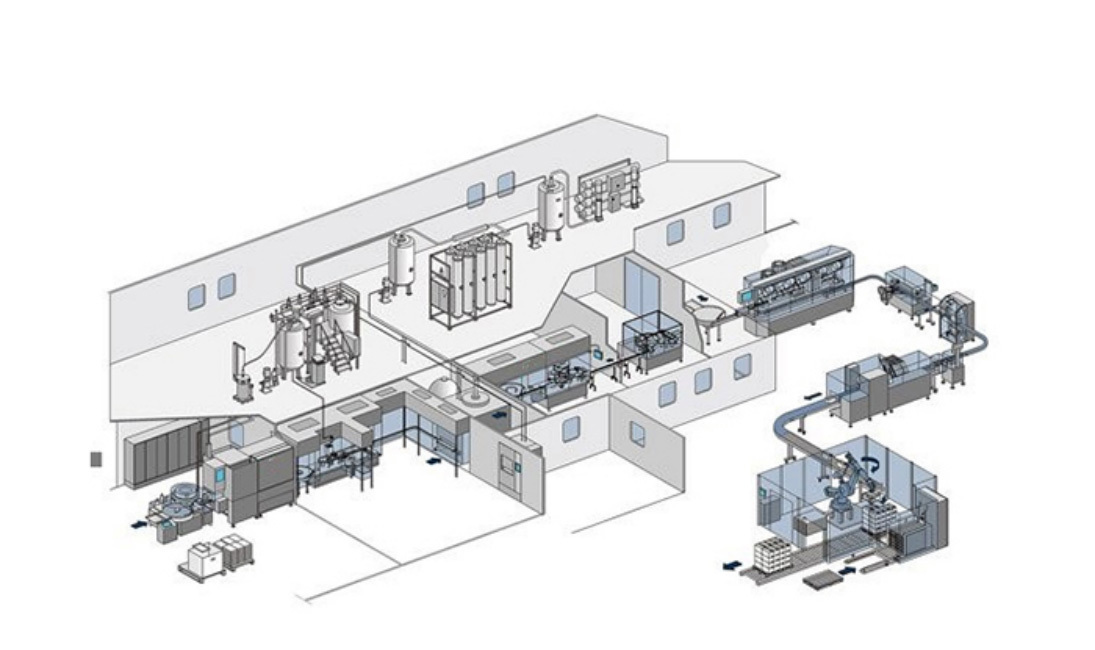
The multi-effect distilled water machine utilizes the principle of multi-stage evaporation and condensation. The purified water that has been fully preheated passes through the first-effect evaporator heated by industrial steam to generate secondary steam. This secondary steam serves as the heating steam for the next effect. The concentrated water from the previous effect is used as the raw water for the subsequent effect and is heated and evaporated again. This process is repeated successively. Finally, the generated steam is condensed by the cooling water, and the non-condensable gases and impurities are removed. The steam condenses in the condenser, thus obtaining high-purity distilled water.
-
1.Three - stage separation with an external spiral structure, including falling - film flash evaporation separation, 180° return force separation, and external spiral centrifugal separation.
2.Double tube - sheet design to prevent cross - contamination. The inner tube - sheet is connected to the outer tube - sheet by expansion.
3.The parts in contact with raw water, cooling water, pure steam, and distilled water are made of stainless steel 316L, and the seals are made of PTFE.
4.A non - condensable gas discharge device is installed.
5.The first - effect evaporator can produce pure steam (optional).
6.We can provide Validation documents include DQ, IQ, OQ. -
Design Pressure(MPa) 0.6 Working Pressure(MPa) 0.3~0.5 WFI output(L/h) 100L/H~5000L/H WFI temperature(℃) 92~99 PH Value 5.0-7.0 Conductivity (at 20 °C) ≤1.0μs/cm Total Organic Carbon (TOC) ≤250ppb(20℃) Bacterial Endotoxin ≤0.125 EU/ml Microbial Limit Escherichia coli, Salmonella, and Pseudomonas aeruginosa shall not be detected The total number of bacteria, molds, and yeasts shall not exceed 2 per 100 ml The total number of bacteria, molds, and yeasts shall not exceed 10 per 100 ml Other indicators of Water for Injection comply with Chinese Pharmacopoeia (2020 Edition) United States Pharmacopeia (USP38) European Pharmacopoeia (EP6.8) -
① Production of sterile preparations such as injections, eye drops, and sterile irrigation solutions.
② Production of biological products and vaccines, such as cell culture medium preparation, buffer dilution, etc.
③ Final cleaning of items in direct contact with sterile products or primary packaging (e.g., vials, stoppers).
④ Cleaning of cleanroom garments in sterile production areas and rinsing of key equipment after sterilization.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Are you manufacturer or trader? Where are your factory located?

We are manufacturer. Our factory is located in Changsha and Suzhou.

What certificate do you have?

We have CE, ISO, ROHS, SGS etc.

What documents will you provide?

Usually we will provide DQ, IQ, PQ, OQ, FAT, SAT, operation manual, maintenance instruction,layout drawing.etc.

What is the production lead time?

Standard 25-60 days after deposit received and technical data confirmed, we can make special arrangement for better shipment if needed;
Standard 60-150 days after deposit received and technical data confirmed based on different machines, we can make special arrangement for better shipment if needed.

How long is your warranty? Can we extend?

12 months after installation and commissioning or 18 moths from the day that the equipment arrived on site. Prevail in the first arrival date.
Warranty can be extended with extra charge.

Have you done similar projects overseas?

Marya’s business map covers more than 60 countries and regions around the world, such as the United States, Russia, Bulgaria, Britain, Portugal, Poland, Switzerland, ltaly, New Zealand, Malta, Moldova, Malaysia, Uzbekistan, lran, Egypt, Saudi Arabia, UAE, Ecuador, Dominica, Panama, Uruguay, Argentina, Tanzania, Zimbabwe, Ethiopia, Indonesia, Bangladesh, and others, and we gained good reputation.
Key words:
Contact MARYA Engineers to Claim Your Free GMP compliant Sterile Water Treatment Solution
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131