Products





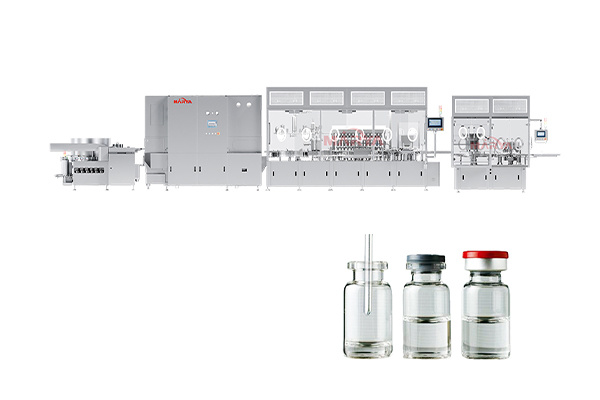
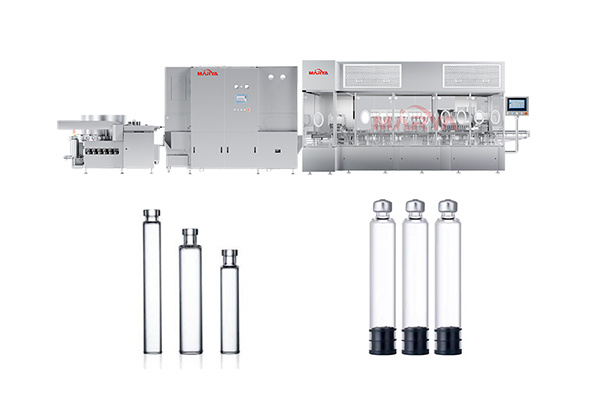
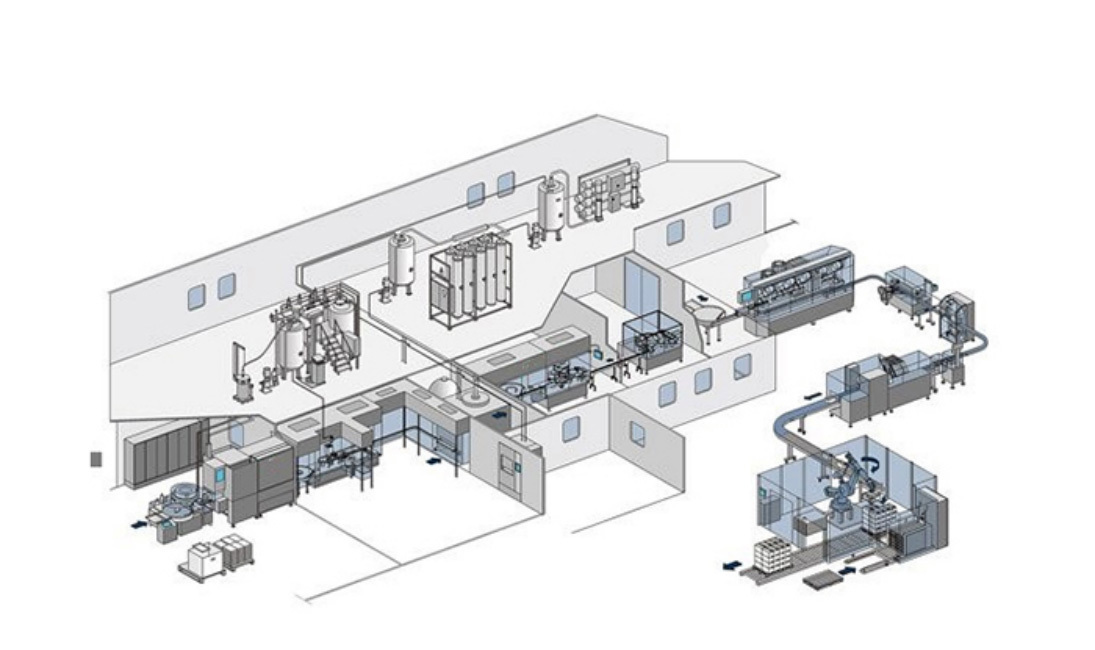
Vial Liquid Filling Sealing Production Line
- Core Advantages
- Core Technology
- Technical Parameters
- Application Scenarios
- Optional Systems
-
Sterility assurance: Equipped with A level laminar flow protection system to ensure a class 100 clean environment;
High-precision filling: Servo-drive can realize ±0.5% high-precision filling, up to 400 pcs/min production capacity;
Integrated modularization: Compact and stable modular design for fully automated operation;
Efficient capping: Fully automatic aluminum cover feeding and capping system, with ≥ 99.5% qualified rate and adjustable strength;
Bottle cleaning: Combines ultrasonic cleaning and high-pressure water jetting, achieving >99.8% cleaning efficiency;
Inert gas replacement: Nitrogen purging before filling to prevent oxidation and degradation of liquid drugs;
High flexibility: Supports for vials (2R~100R), suitable for small batch and multi-variety production needs;
Intelligent control:
· Siemens touch screen: Intelligent PLC-based control system with touchscreen operation and data traceability;
· Intelligent quality control: Intelligent visual inspection for real-time monitoring of filling/sealing quality, automatic rejection of defective products and full-process traceability. -
Layout Design: The whole line can adopt straight-line layout or wall-to-wall L-shaped layout to reduce the risk of cross-contamination and ensure aseptic level;
Washing Process: Ultrasonic cleaning + compressed air purging to remove particles and microorganisms, complying with pharmacopoeial cleanliness standards;
Drying & Sterilization: The heat in the hot air circulation tunnel oven is evenly distributed, ensuring effective depyrogenation;
Filling Process: Supporting the filling of liquids, semi-solids (gel), and high-viscosity formulations (e.g., syrups, suspensions);
Stoppering Process: High-precision mechanical stoppering with ≥99.5% success rate, leak-proof and contamination-resistant;
Capping Process: Adopting stable and efficient single knife rolling technology, using mechanical capping device to cover the aluminum cap tightly on the mouth of the vial;
Inspection Process: It can be equipped with light inspection machine for vials and integrated visual inspection system to monitor the quality of filling and sealing in real time and automatically reject unqualified products.
-
Specifications 1ml~100ml(2R~100Rvial bottles) Filling Accuracy ≤±0.5%(according to drug characteristics and filling volume) Various Filling Pumps glass pump, metal pump, peristaltic pump, ceramic pump Production Speed 10~400 bottles/min (programmable adjustment) Production Capacity 1000-24000BPH Filling Heads 1-12, to be selected according to the output Capping Qualified Rate ≥99.5% Power Requirement 380V, 50/60Hz Internal Environment Laminar flow air cleanliness level 100 -
Aseptic injections: antibiotics, chemotherapeutic drugs, biological protein preparations and other aqueous needle filling and capping.
Freeze-drying preparation: linkage freeze-drying machine to realize filling → freeze-drying → plugging → capping the whole process of automation.
Oral liquid and health care products: Chinese medicine oral liquid, probiotic liquid, collagen beverages encapsulation.
Vaccine production: Vial filling of inactivated vaccines, mRNA vaccines and other biological preparations;
CRO/CDMO: small batch flexible production and process validation for clinical trial drugs. -
▸ Sterility Assurance System
Optional o-RABS/isolation protection system or aseptic isolator system;
Optional sterilization temperature real-time display & printing system;
Optional online sterilization function for the cooling section of the hot air tunnel oven;
Optional CIP/SIP system: to ensure compliance with GMP requirements, guaranteeing residue-free cleaning.▸ Intelligent Inspection System
Online monitoring system: It can monitor the key factors affecting product quality (such as dust particles, planktonic bacteria, wind speed, wind pressure, etc.);Electronic Batch Records (EBR): It complies with FDA 21 CFR Part 11 and automatically generates audit trail reports.
▸ Automated Control System
Intelligent error-proofing: no bottle no filling, no bottle no stoppering, no bottle no capping and auto-stop function for bottle squeeze, vial missing , cap missing , or stopper missing;Aluminum dust extraction: It can remove aluminum particles generated during capping, reducing the risk of environmental pollution.
▸ Quality Tracing System
Audit trail system: Equipped with Siemens industrial control machine & WinCC system to realize complete production process traceability;Standard real-time display of sterilization temperature & printing system.
▸ Customization Services
Modular design, flexible configuration.Supports special process customization.
Provides validation services support.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Are you manufacturer or trader? Where are your factory located?

We are manufacturer. Our factory is located in Changsha and Suzhou.

What certificate do you have?

We have CE, ISO, ROHS, SGS etc.

What documents will you provide?

Usually we will provide DQ, IQ, PQ, OQ, FAT, SAT, operation manual, maintenance instruction,layout drawing.etc.

What is the production lead time?

Standard 25-60 days after deposit received and technical data confirmed, we can make special arrangement for better shipment if needed;
Standard 60-150 days after deposit received and technical data confirmed based on different machines, we can make special arrangement for better shipment if needed.

How long is your warranty? Can we extend?

12 months after installation and commissioning or 18 moths from the day that the equipment arrived on site. Prevail in the first arrival date.
Warranty can be extended with extra charge.

Have you done similar projects overseas?

Marya’s business map covers more than 60 countries and regions around the world, such as the United States, Russia, Bulgaria, Britain, Portugal, Poland, Switzerland, ltaly, New Zealand, Malta, Moldova, Malaysia, Uzbekistan, lran, Egypt, Saudi Arabia, UAE, Ecuador, Dominica, Panama, Uruguay, Argentina, Tanzania, Zimbabwe, Ethiopia, Indonesia, Bangladesh, and others, and we gained good reputation.
Key words:
Vial liquid filling line
vial filling machine
vial filling line
vial filling production line
Liquid Vial Filling Machines
vial filling machine manufacturer
Previous:
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131