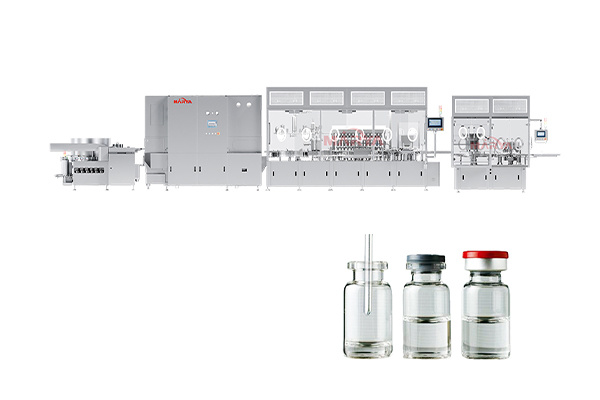
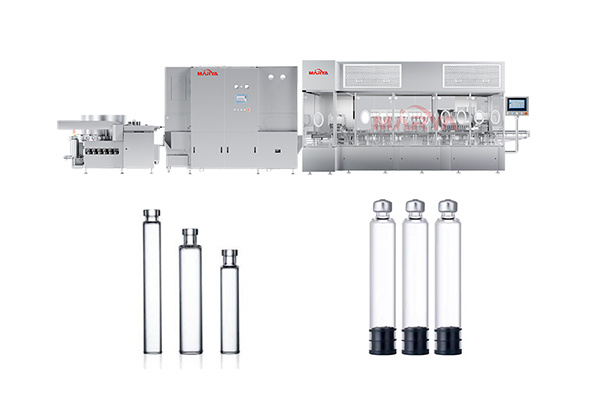
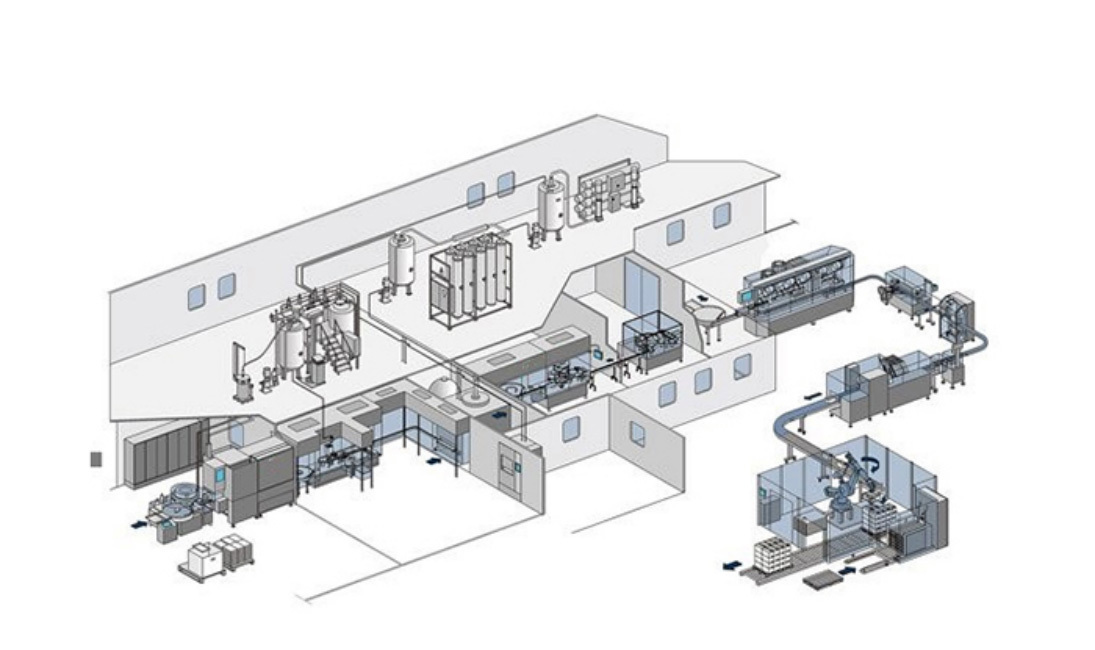
Products





Pharmaceutical Liquid Preparation System
- Core Features
- Functions
- Compliance & Standards
- Application Scenarios
- Technical Parameter
-
1. CIP/SIP-ready for easy cleaning and sterilization.
2. The tank body adopts a bottom-mounted magnetic drive agitator, which has no risk of leakage and more uniform stirring.
3. The system adopts a 3D module design, which is compact, beautiful and saves floor space.
4. Flexible formula management, electronic signature, electronic records and audit tracking functions.
5. The system can be equipped with online detection instruments such as PH, O3, conductivity, etc., and key process parameters can be controlled in production.
6. Key components are all high-end European and American brands with stable and reliable performance.
7. Fully compatible with our vial filling, ampoule filling, or syringe filling lines. Seamless integration ensures consistent fluid transfer, minimized contamination risk, and streamlined operations.
-
Basic Functions Optional Functions CIP (Clean in place) Vacuuming inside tank SIP (Sanitize in Place) Nitrogen protection Hot water circulation On-line integrity testing Multi-level user access rights PH automatic control Recipe management WFI instant cooling Audit trail Real-time data printing Electronic record Pipeline temperature control -
Designed and manufactured according to GMP guidelines
CE marked
Full documentation support: FAT/SAT, IQ/OQ protocols
21 CFR Part 11 compliant (for software & data integrity) -
Biological preparations (e.g., vaccines, antibodies)
Injectable solutions
Eye drops
Aesthetic medicine products -
Name High Volume Preparation System Small Volume Preparation System Tank Volume(L) 3000/5000/10000 50/300/500/1000 Tank Functions Pre-preparation→Preparation Pre-preparation→Preparation→Storage Working Volume (L) 200~1000/500~3000/
800~5000/1000~10000
10~50/50~300/100~500 Tank Material Inner 316L, Insulation shell 304 Inner 316L, Insulation shell 304 Design Pressure(MPa) -0.1~0.4 -0.1~0.4 Design Temperature(℃) -10~150 -10~150 Type of mixing Magnetic drive stirrer Magnetic drive stirrer Volume measurement Load sensor Load sensor
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Are you manufacturer or trader? Where are your factory located?

We are manufacturer. Our factory is located in Changsha and Suzhou.

What certificate do you have?

We have CE, ISO, ROHS, SGS etc.

What documents will you provide?

Usually we will provide DQ, IQ, PQ, OQ, FAT, SAT, operation manual, maintenance instruction,layout drawing.etc.

What is the production lead time?

Standard 25-60 days after deposit received and technical data confirmed, we can make special arrangement for better shipment if needed;
Standard 60-150 days after deposit received and technical data confirmed based on different machines, we can make special arrangement for better shipment if needed.

How long is your warranty? Can we extend?

12 months after installation and commissioning or 18 moths from the day that the equipment arrived on site. Prevail in the first arrival date.
Warranty can be extended with extra charge.

Have you done similar projects overseas?

Marya’s business map covers more than 60 countries and regions around the world, such as the United States, Russia, Bulgaria, Britain, Portugal, Poland, Switzerland, ltaly, New Zealand, Malta, Moldova, Malaysia, Uzbekistan, lran, Egypt, Saudi Arabia, UAE, Ecuador, Dominica, Panama, Uruguay, Argentina, Tanzania, Zimbabwe, Ethiopia, Indonesia, Bangladesh, and others, and we gained good reputation.
Key words:
Pharmaceutical Liquid Preparation System
Pharmaceuitical Preparation System
GMP Cell Drug Preparation Station
Preparation System
Previous:
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131