Products




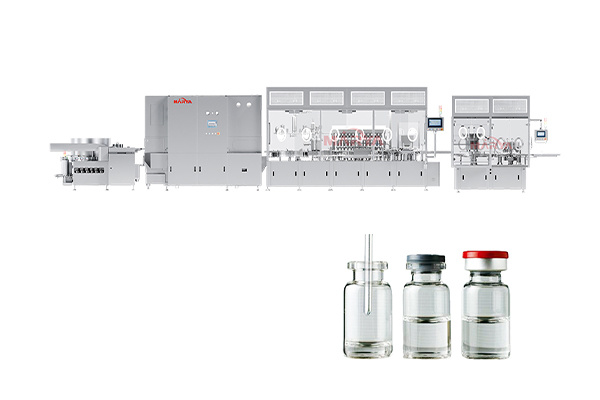
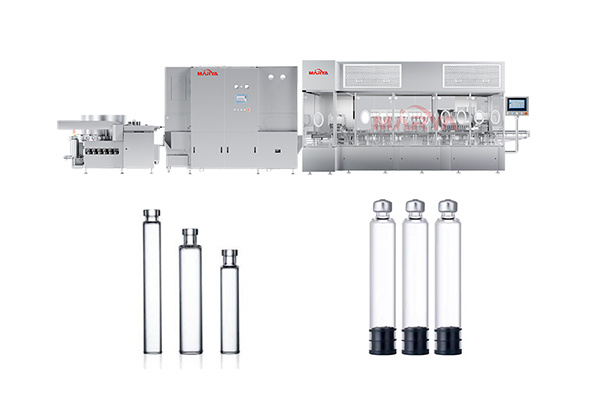
Vial Powder Filling Sealing Production Line
- Core Advantages
- Core Technology
- Technical Parameters
- Application Scenarios
- Optional Systems
-
High filling accuracy: Up to ±3% filling deviation, fully meets GMP standards
Fully automated operation: Minimizes manual intervention to reduce contamination risks
Sterility assurance: Isolator system ensures clean production environment
Production flexibility: Quick changeover to meet the needs of multiple specifications
Stability and efficiency: Servo-driven system ensures continuous stable operation
Feeding system: Multiple sterile material feeding options, in line with GMP requirements
Intelligent control: PLC + touchscreen for fully automated monitoring
Compact design: Space-saving footprint and reasonable layout
-
Sterilization Oven: Evenly distributed heat in the hot air circulation tunnel oven with good depyrogenation effect;
Powder Filling: High-precision screw is used to realize precise filling of sterile powder, eliminating secondary contamination;
Vial Positioning: Photoelectric sensors and mechanical positioning systems are used to ensure precise vial positioning during filling;
Sealing & Capping: Automatically completes aluminum cap sealing/rubber plug capping post-filling;
Online Inspection: Integrated in-line weighing for 100% quality control.
-
Specifications 5ml~50mlvial bottles (according to per customer’s requirement) Production Capacity 6000-30000BPH Filling Heads 1-4, to be selected according to the output Filling Accuracy ≤±3%(according to drug characteristics and filling volume) Capping Qualified Rate ≥99.9% FillingSpeed 100 bottles/min (adjustable according to demand) Power Requirement 380V,50-60Hz Air Source Requirement 0.6-0.8 MPAclean compressed air Clean Level Meet GMP Level A requirements -
Pharmaceutical industry: For filling antibiotics, freeze-dried powder injection, vaccines and other powdered drugs.
Biological products: For aseptic filling of high-end biological products, such as biological preparations and cell cultures.
Cosmetic industry: For powder filling of high-end skin care products, such as mask powder, essence powder, etc.
Food industry: Suitable for powder filling of functional foods and nutritional supplements.
-
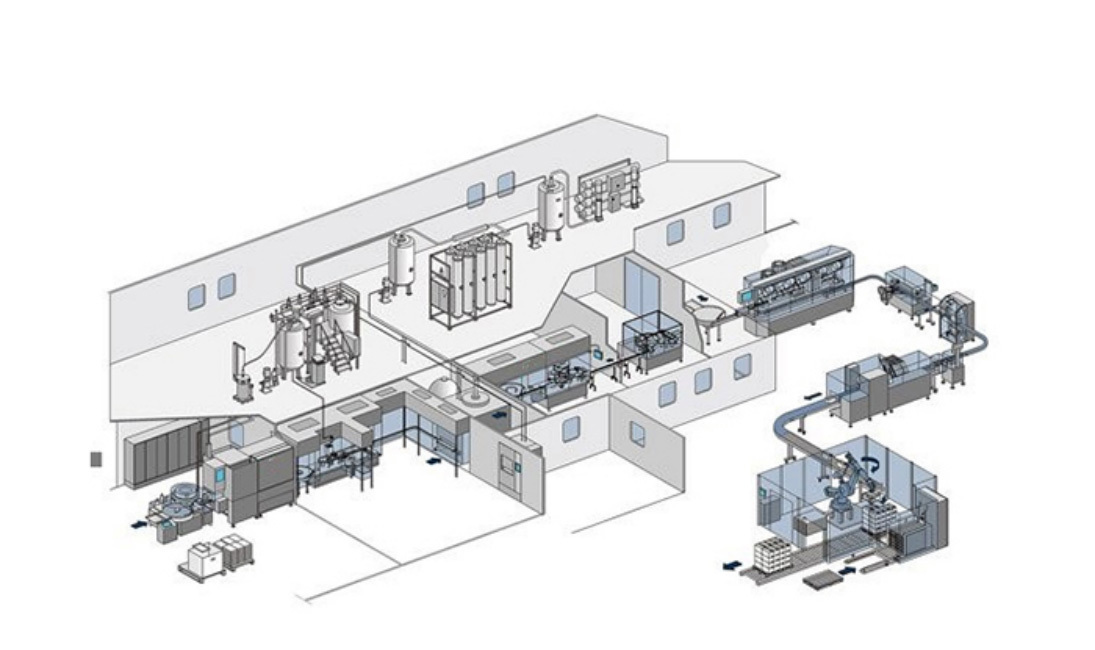
▸ Sterility Assurance System
Optional o-RABS/isolation protection system or aseptic isolator system;
Optional sterilization temperature real-time display & printing system;
Optional online sterilization function for the cooling section of the hot air tunnel oven;
Optional CIP/SIP system: to ensure compliance with GMP requirements, guaranteeing residue-free cleaning.▸ Intelligent Inspection System
Online weighing system: Real-time monitoring of the weight of each bottle of powder to ensure filling accuracy.Online monitoring system: It can monitor the key factors affecting product quality (such as dust particles, planktonic bacteria, wind speed, wind pressure, etc.);
▸ Automated Control System
Servo automatic feeding: Automatic feeding and filling of powder and less manual operation.Intelligent error-proofing: no bottle no filling, no bottle no stoppering, no bottle no capping and auto-stop function for bottle squeeze or vial missing;
Aluminum dust extraction: It can remove aluminum particles generated during capping, reducing the risk of environmental pollution.
▸ Quality Tracing System
Supervisory code system: Equipped with QR codes or bar codes for complete product traceability.Standard real-time display of sterilization temperature & printing system.
▸ Customization Services
Modular design, flexible configuration.Supports special process customization.
Provides validation services support.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Are you manufacturer or trader? Where are your factory located?

We are manufacturer. Our factory is located in Changsha and Suzhou.

What certificate do you have?

We have CE, ISO, ROHS, SGS etc.

What documents will you provide?

Usually we will provide DQ, IQ, PQ, OQ, FAT, SAT, operation manual, maintenance instruction,layout drawing.etc.

What is the production lead time?

Standard 25-60 days after deposit received and technical data confirmed, we can make special arrangement for better shipment if needed;
Standard 60-150 days after deposit received and technical data confirmed based on different machines, we can make special arrangement for better shipment if needed.

How long is your warranty? Can we extend?

12 months after installation and commissioning or 18 moths from the day that the equipment arrived on site. Prevail in the first arrival date.
Warranty can be extended with extra charge.

Have you done similar projects overseas?

Marya’s business map covers more than 60 countries and regions around the world, such as the United States, Russia, Bulgaria, Britain, Portugal, Poland, Switzerland, ltaly, New Zealand, Malta, Moldova, Malaysia, Uzbekistan, lran, Egypt, Saudi Arabia, UAE, Ecuador, Dominica, Panama, Uruguay, Argentina, Tanzania, Zimbabwe, Ethiopia, Indonesia, Bangladesh, and others, and we gained good reputation.
Key words:
Vial Powder Production Line
vial powder filling machine
injectable vial powder filling machine
dry powder vial filling machine
vial powder filling machine manufacturer
pharmaceutical powder filling machine
vial auger powder filling machine
Auger powder filling Machine
vial powder filling line
powder filling machine
sterile vial powder filling line
syrup powder filling line
Vial powder injection production line
:Next
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131