Products




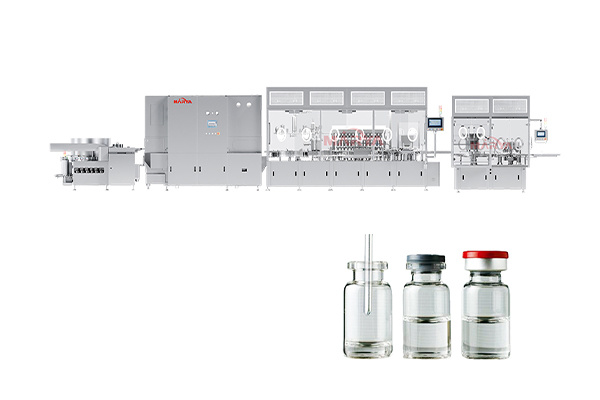
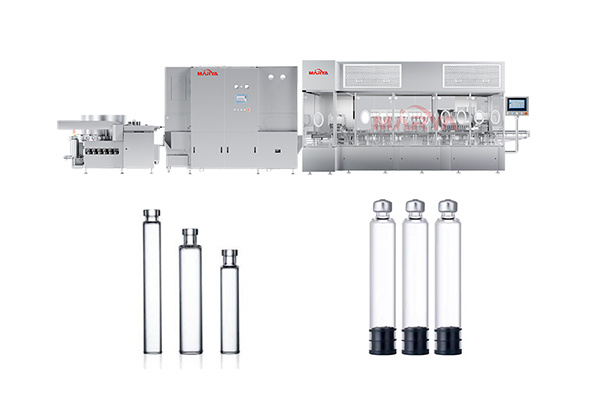
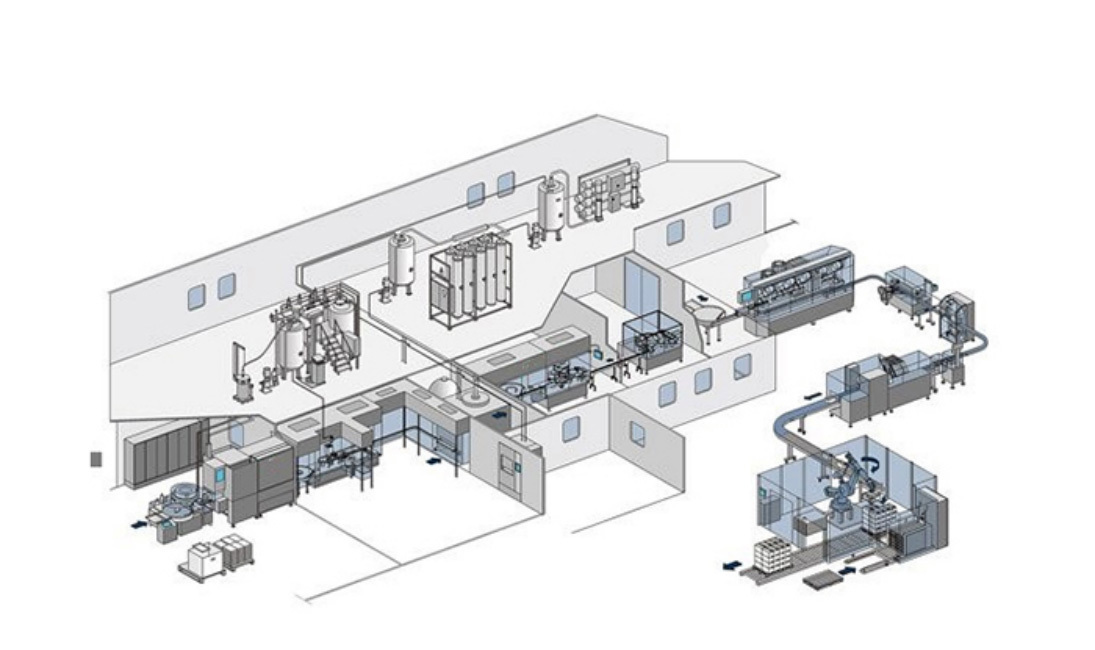
RTU Vial Syringe Cartridge Filling Machine
- Core Advantages
- Core Technology
- Technical Parameters
- Application Scenarios
- Optional Systems
-
Multi-container compatibility:
· Fast changeover: Supports multiple specification containers (vials, pre-filled syringes, cartridges), changeover time for specification parts ≤ 60 minutes.
· Adaptive clamping: High-precision servo-controlled clamps prevent scratches and deformation.
Aseptic operation: The whole filling and stoppering process is aseptic, preventing microbial contamination.
Ultra-precision filling: Ceramic pumps, peristaltic pumps, mass flow meters achieve ±1% accuracy. And it’s adaptable to diverse drug formulations and packaging materials.
Easy integration: Modular design interfaces seamlessly with front and rear equipment, allowing future upgrades.
Easy maintenance: Quick-disassembly structure reduces cleaning time and maintenance costs.
Flexible production mode: Supports single batch and continuous production, etc.
High stability: Ensures consistent quality during long operation periods.
Regulatory compliance: Meets GMP, FDA 21CFR part11 and other regulations.
Intelligent features:
· Touch screen adjustment of filling volume and plugging depth with servo motor independent pump control.
· Full traceability: MES system records data and supports electronic batch records.
· Safety: detection of plug lack, overload protection, abnormal condition alarm ensure 100% stoppering. -
Sterility guarantee: ISO Class 5 isolator + VHP online sterilization (≥6-log decontamination)
Pollution-free filling: Pre-filled syringe filling heads with suspension positioning technology, avoiding liquid splashing and reducing the risk of cross-contamination by 90%.
High-precision sealing:
· Syringes: particle-free rubber stopper press-fit, passing USP<1207> sealing test
· Vials/Cartridges: Electromagnetic induction capping, with ±1% torque control accuracy, ensuring leak-proof aluminum seal
Antioxidant protection: Equipped with nitrogen filling system to meet the antioxidant process requirements during drug filling and storage;
Seal detection: High sensitivity negative pressure detection; leakage resolution up to 0.05ccm; real-time rejection of micro-leakage products. -
Items Parameters Applicable Containers Vial(2R~30R), pre-filled syringe(1ml~10ml), cartridge (1.5-3ml) Filling Accuracy ±0.2% (syringe) /±0.5% (vial/cartridge) Production Speed Vial: 200 bottles/min;
Pre-filled syringe/Cartridge: 350 pcs/min (maximum speed)
Cleanliness Class ISO Level 5 (Level A environment) Power Requirement 380V±10%, 50/60Hz Compressed Air Requirement 0.7MPa, no oil and water (dew point ≤-40℃) -
Biologics: ready-to-use dispensing of monoclonal antibodies, dual antibodies, ADC drugs, cell therapy solutions.
Vaccine field: pre-filled syringe encapsulation of mRNA vaccines, recombinant protein vaccines.
High-end medical aesthetics: aseptic cartridge filling for hyaluronic acid, Poly-L-lactic acid treatments(“Youth Boosters”), collagen.
Chronic disease medication: automated production of pen cartridges for insulin, GLP-1 receptor agonists.
Emergency medicines: ready-to-use packaging of epinephrine pre-filled syringes and anticoagulants. -
· Isolator + RTP interface: optional C-RABS and O-RABS protection to realize aseptic operation in Class A environment.
· Online sterilization plunger pump can be configured to realize CIP/SIP function of filling system.
· Lyophilizer linkage module: Directly interface with lyophilization process after filling of vials, reducing the intermediate exposure link.
· Automatic siliconization system: silicone oil spraying on the inner wall of pre-filled syringe; uniformity CV≤3%, reducing injection thrust.
· Particle monitoring system: Online monitoring of the number of particles ≥ 0.5μm in the filling area; real-time data warning.
· AGV logistics integration: Seamless interface with intelligent warehouse system to realize unmanned material turnover.
· Adopt PMS online monitoring system, achieving real-time monitoring of sterile production environment and data traceability.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Are you manufacturer or trader? Where are your factory located?

We are manufacturer. Our factory is located in Changsha and Suzhou.

What certificate do you have?

We have CE, ISO, ROHS, SGS etc.

What documents will you provide?

Usually we will provide DQ, IQ, PQ, OQ, FAT, SAT, operation manual, maintenance instruction,layout drawing.etc.

What is the production lead time?

Standard 25-60 days after deposit received and technical data confirmed, we can make special arrangement for better shipment if needed;
Standard 60-150 days after deposit received and technical data confirmed based on different machines, we can make special arrangement for better shipment if needed.

How long is your warranty? Can we extend?

12 months after installation and commissioning or 18 moths from the day that the equipment arrived on site. Prevail in the first arrival date.
Warranty can be extended with extra charge.

Have you done similar projects overseas?

Marya’s business map covers more than 60 countries and regions around the world, such as the United States, Russia, Bulgaria, Britain, Portugal, Poland, Switzerland, ltaly, New Zealand, Malta, Moldova, Malaysia, Uzbekistan, lran, Egypt, Saudi Arabia, UAE, Ecuador, Dominica, Panama, Uruguay, Argentina, Tanzania, Zimbabwe, Ethiopia, Indonesia, Bangladesh, and others, and we gained good reputation.
Key words:
Prefilled Syringe Filling Machine
RTU Syringe Cartrige Vial Filling Machine
Pre-Filled Syringe Filling Line
Pfs Machine
Plastic PFS filling machine
PFS filling machine
RTU vial filling machine
RTU syringe filling machine
RTU cartridge filling machine
vial filling machine manufacturer
vaccine vial filling machine
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131